Synthesis of 3-benzylidenetetrahydrofurans: Tf2O-catalyzed hydroxylation/cyclization of cyclopropanemethanols with DMSO†
Abstract
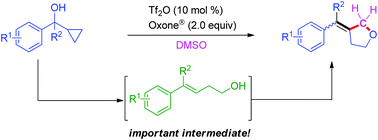
A formal hydroxylation/cyclization of cyclopropanemethanols with DMSO is described, which involves isomerization and cyclization under Tf2O catalysis. This reaction undergoes ring-opening of the cyclopropane moiety to generate homoallylic alcohols, which react with DMSO to produce 3-benzylidenetetrahydrofurans. With various substituted groups on cyclopropanemethanols the reactions proceed smoothly and the desired 3-benzylidenetetrahydrofurans are obtained in moderate to good yields.


 Please wait while we load your content...
Please wait while we load your content...