Reduction of dioxygen to water by a Co(N2O2) complex with a 2,2′-bipyridine backbone†
Abstract
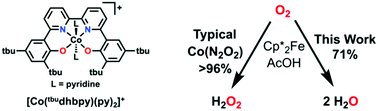
We report a Co-based complex for the reduction of O2 to H2O utilizing decamethylferrocene as chemical reductant and acetic acid as a proton donor in methanol solution. Despite structural similarities to previously reported Co(N2O2) complexes capable of catalytic O2 reduction, this system shows selectivity for the four-electron/four-proton reduction product, H2O, instead of the two-electron/two-proton reduction product, H2O2. Mechanistic studies show that the overall rate law is analogous to previous examples, suggesting that the key selectivity difference arises in part from increased favorability of protonation at the distal O position of the key intermediate Co(III)-hydroperoxide, instead of the proximal one. Interestingly, no product selectivity dependence is observed with respect to the presence of pyridine, which is proposed to bind trans to O2 during catalysis.


 Please wait while we load your content...
Please wait while we load your content...