Catalytic asymmetric aldehyde prenylation and application in the total synthesis of (−)-rosiridol and (−)-bifurcadiol†
Abstract
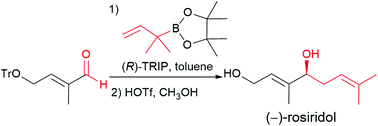
Chiral phosphoric acid-catalyzed asymmetric aldehyde prenylation has been established using an α,α-dimethyl allyl boronic ester. The transformation provides expedient access to a wide array of aryl, heteroaryl, aryl-substituted alkenyl and primary and secondary aliphatic homoprenyl alcohols with excellent asymmetric induction. The utility of this asymmetric catalysis strategy has been demonstrated through a short and efficient total synthesis of the two natural products (−)-rosiridol and (−)-bifurcadiol.


 Please wait while we load your content...
Please wait while we load your content...