Plasmid-loadable magnetic/ultrasound-responsive nanodroplets with a SPIO-NP dispersed perfluoropentane core and lipid shell for tumor-targeted intracellular plasmid delivery
Abstract
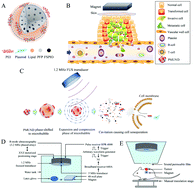
Using ultrasound activating contrast agents to induce sonoporation is a potential strategy for effective lesion-targeted gene delivery. Previous reports have proven that submicron nanodroplets have a better advantage than microbubbles in that they can pass through tumor vasculature endothelial gaps by passive targeting; however, they cannot achieve an adequate dose in tumors to facilitate ultrasound-enhanced gene delivery. Additionally, a few studies focused on delivering macromolecular genetic materials (i.e. overexpression plasmid and CRISPR plasmid) have presented more unique advantages than small-molecular genetic materials (i.e. miRNA mimics, siRNA and shRNA etc.), such as enhancing the expression of target genes with long-term effectiveness. Thereby, we constructed novel plasmid-loadable magnetic/ultrasound-responsive nanodroplets, where superparamagnetic iron oxide nanoparticle dispersed perfluoropentane was encapsulated with lipids to which plasmids could be adhered, and branched polyethylenimine was used to protect the plasmids from enzymolysis. Furthermore, in vitro and in vivo studies were performed to verify the magnetic tumor-targeting ability of the plasmid-loadable magnetic/ultrasound-responsive nanodroplets and focused ultrasound enhanced intracellular plasmid delivery. The plasmid-loadable magnetic/ultrasound-responsive nanodroplets, carrying 16–19 plasmids per droplet, had desirable diameters less than 300 nm, and integrated the merits of excellent magnetic targeting capabilities and phase transition sensitivity to focused ultrasound. Under programmable focused ultrasound exposure, the plasmid-loadable magnetic/ultrasound-responsive nanodroplets underwent a phase-transition into echogenic microbubbles and the subsequent inertial cavitation of the microbubbles achieved an ∼40% in vitro plasmid delivery efficiency. Following intravenous administration, T2-weighted magnet resonance imaging, scanning electron microscopy and inductively coupled plasma optical emission spectrometry of the tumors showed significantly enhanced intratumoral accumulation of the plasmid-loadable magnetic/ultrasound-responsive nanodroplets under an external magnetic field. And a GFP ELISA assay and immunofluorescence staining indicated that focused ultrasound-induced inertial cavitation of the plasmid-loadable magnetic/ultrasound-responsive nanodroplets significantly enhanced the intracellular delivery of plasmids within the tumor after magnet-assisted accumulation, while only lower GFP levels were observed in the tumors on applying focused ultrasound or an external magnet alone. Taken together, utilizing the excellent plasmid-loadable magnetic/ultrasound-responsive nanodroplets combined with magnetism and ultrasound could efficiently deliver plasmids to cancer cells, which could be a potential strategy for macromolecular genetic material delivery in the clinic to treat cancer.

 Please wait while we load your content...
Please wait while we load your content...