Acute and 30-day oral toxicity studies of a novel coccidiostat – ethanamizuril
Abstract
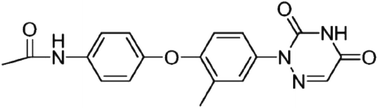
Ethanamizuril is a novel triazine compound that exhibits remarkable anticoccidial activity. Owing to its pharmacological properties, this study was conducted to evaluate the acute and 30-day oral toxicity of ethanamizuril. In the acute study, ethanamizuril was administered once by oral gavage to mice and rats. The calculated LD50 values for mice and rats were 5776 and 4743 mg per kg b.w, respectively, but the LD50 value for male rats was higher than that of female rats. In the subchronic study, male and female rats were fed with diets supplemented with 0, 20, 60 or 120 mg kg−1 ethanamizuril for 30 days. Treatment related clinical signs of alopecia on the back and neck of the animals were observed in the 60 and 120 mg kg−1 dose groups from the third week of treatment. Significant differences in haematological and biochemical parameters as well as organ-to-body weight ratios were detected between the 60 and 120 mg kg−1 groups. Histopathological observations revealed that 60 and 120 mg kg−1 ethanamizuril could induce focal hepatocellular necrosis and split phase. Slight renal tubule protein casts in the kidneys and alveolar wall thickening in the lungs were also observed in the high dose groups of both genders. The dietary no-observed-adverse-effect level (NOAEL) of ethanamizuril for 30 days was 20 mg kg−1 feed.


 Please wait while we load your content...
Please wait while we load your content...