Spectroscopy and dynamics of a HOF and its molecular units: remarkable vapor acid sensing†
Abstract
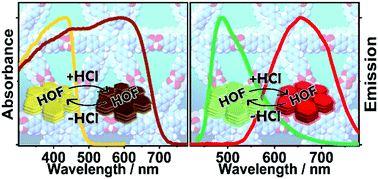
Hexaazatriphenylene (HAT) derivatives are being used for the fabrication of hydrogen-bonded organic frameworks (HOFs). Here, we report on studies of two HAT derivatives: CBPHAT and its butylated partner, CBPHAT-C4H9, in N,N-dimethylformamide (DMF) solutions and solid state. CBPHAT displays different species and no planar conformations when interacting with DMF. CBPHAT-C4H9 undergoes an intramolecular charge transfer (ICT) reaction in 50 ps, while for CBPHAT, the ICT reaction is faster (∼4.5 ps). Their emission lifetimes in DMF solution change from 50 ps to 2.3 ns. The slower relaxation of CBPHAT-C4H9 without oxygen quenching involves three long living states (∼2, ∼18 and ∼100 μs). The CBPHAT-1a HOF (crystals) exhibit both proton and charge transfer reactions (60 ps), leading to different emitters with fluorescence lifetimes spanning from 100 ps to 2.66 ns. This HOF shows a remarkably strong response to HCl vapors in a reversible way in ambient conditions. This ability is reflected in its absorption and emission spectra, making it a new smart sensor of acid gas. The provided theoretical and experimental results should trigger more research on this family and related HOFs to further advance its science.


 Please wait while we load your content...
Please wait while we load your content...