Carrier-free self-built aspirin nanorods as anti-aggregation agents towards alpha-crystallin-derived peptide aggregates: potential implications in non-invasive cataract therapy†
Abstract
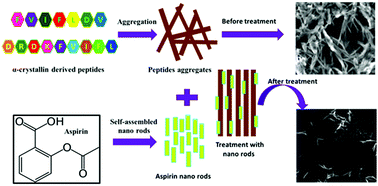
The aggregation of the α-crystallin protein is the pathological hallmark of cataract. In the current work, peptide fragments derived from native α-crystallin were synthesized and explored as a peptide-based crystallin aggregation model towards cataract. The anti-aggregation potential of aspirin was evaluated towards these peptide-generated aggregates as well as towards the α-crystallin aggregate. The results demonstrated that aspirin had the capacity to inhibit crystallin and crystallin-derived peptide aggregation and could act as a potential therapeutic agent in mitigating cataract. Computational studies were also carried out to study the interaction between the model peptides and aspirin. The results revealed the existence of molecular interactions between the peptides and aspirin, which had a significant impact on the secondary structure of the peptides and potentially modulated their assembly and aggregation behavior. The formation of self-built aspirin nanorods was also explored and their ability to inhibit the aggregation of model cataract peptides and α-crystallin aggregation was validated. These findings open up the possibility of using small molecule-based nanotherapeutics for cataract merely through topical applications, which can be beneficial to cataract patients.


 Please wait while we load your content...
Please wait while we load your content...