Electronic doping-enabled transition from n- to p-type conductivity over Au@CdS core–shell nanocrystals toward unassisted photoelectrochemical water splitting†
Abstract
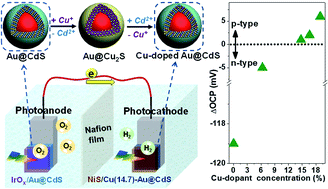
Here we show a novel strategy for tailoring the synergistic electrical properties of metal@semiconductor hybrid nanocrystals (HNCs) based on cation exchange-enabled electronic doping. As a demonstration, the conductive nature of Au@CdS core–shell HNCs was changed from n- to p-type by introducing Cu dopants into the CdS shell. The dependence of the conductivity type on the dopant concentration in the HNCs is disclosed by combined photoelectrochemical (PEC) studies. Moreover, a tandem PEC cell consisting of an undoped Au@CdS photoanode and a Cu-doped Au@CdS photocathode respectively modified with cocatalysts is fabricated, which displayed stable H2 and O2 evolution in unassisted PEC overall water splitting.


 Please wait while we load your content...
Please wait while we load your content...