Doping and phase segregation in Mn2+- and Co2+-doped lead halide perovskites from 133Cs and 1H NMR relaxation enhancement†
Abstract
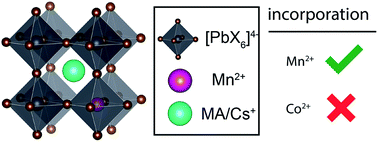
Lead halide perovskites belong to a broad class of compounds with appealing optoelectronic and photovoltaic properties. Doping with transition metal ions such as Mn2+ and Co2+ has recently been reported to substantially enhance luminescence and stability of these materials. However, so far atomic-level evidence for incorporation of the dopants into perovskite phases has been missing. Here, we introduce a general and straightforward method for confirming the substitutional doping of bulk perovskite phases with paramagnetic dopants. Using 133Cs and 1H solid-state MAS NMR relaxation measurements we provide for the first time direct evidence that, consistent with current understanding, Mn2+ is incorporated into the perovskite lattice of CsPbCl3 and CsPbBr3 and does not form clusters. We also show that, contrary to current conviction, Co2+ is not incorporated into the perovskite lattice of MAPbI3.
- This article is part of the themed collection: International Year of the Periodic Table : From Pb and Sn Perovskites to the Next Generation


 Please wait while we load your content...
Please wait while we load your content...