Selective assembly of N1- and N2-alkylated 1,2,3-triazoles via copper-catalyzed decarboxylative cycloaddition of alkynyl carboxylic acids with ethers and azidotrimethylsilane†
Abstract
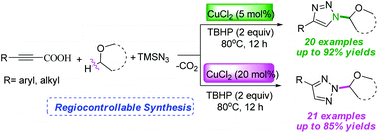
A convenient and practical copper-catalyzed three component protocol has been developed for the selective assembly of N1- and N2-alkylated 1,2,3-triazoles from readily available alkynyl carboxylic acids, azidotrimethylsilane (TMSN3) and ethers. The present reaction can be achieved by decarboxylative cycloaddition of alkynyl carboxylic acids with TMSN3 and ethers, which is an efficient and selective approach to access a number of N1- and N2-alkylated 1,2,3-triazoles in moderate to good yields.


 Please wait while we load your content...
Please wait while we load your content...