An efficient approach to access 1,1,2-triarylethanes enabled by the organo-photoredox-catalyzed decarboxylative addition reaction†
Abstract
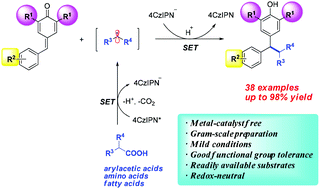
An efficient protocol for the synthesis of 1,1,2-triarylethanes and 2,2-diarylethylamine with biological and pharmacological potential via organo-photoredox-catalyzed decarboxylative 1,6-conjugate addition of carboxylic acids to para-quinone methides has been developed. Different from the traditional transition-metal catalyzed multi-step synthetic strategy, our process involves a photoredox-promoted free radical pathway. A variety of structurally diverse products can be easily obtained under metal-free conditions in good to excellent yields (38 examples, up to 98% yield) in one step.


 Please wait while we load your content...
Please wait while we load your content...