1T-phase MoS2 quantum dots as a superior co-catalyst to Pt decorated on carbon nitride nanorods for photocatalytic hydrogen evolution from water†
Abstract
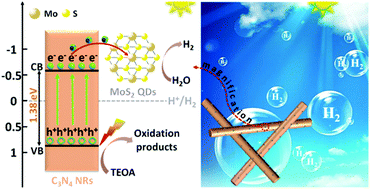
Molybdenum disulfide (MoS2) has been confirmed to be a promising non-precious-metal co-catalyst for photocatalytic hydrogen (H2) evolution; however, its low-density of active sites and poor electron transfer efficiency have essentially limited its photocatalytic properties. Here we report that 1T-MoS2 quantum dots (QDs) can exceed the performance of noble metals like Pt as co-catalysts in assisting photocatalytic H2 evolution upon forming a heterostructure with C3N4 nanorods (denoted as 1T-MoS2@C3N4 NRs). The presence of 1T-MoS2 QDs is found to improve light harvesting, enhance electronic conductivity as well as boost the density of active sites, resulting in an excellent light absorption range up to the near-infrared (NIR) region and a highly efficient spatial charge separation and transfer process. As a result, the optimized 1T-MoS2@C3N4 NR composite (5.0 wt%) exhibits an extraordinary photocatalytic H2 production rate of 565 μmol h−1 g−1 under simulated solar light irradiation, obviously higher than that of noble metal Pt loaded C3N4 NRs (318 μmol h−1 g−1). Moreover, the 1T-MoS2@C3N4 NR composites exhibit good stability in the cyclic runs for photocatalytic H2 production. This study indicates that the highly active MoS2 as a co-catalyst is highly promising as a substitute for Pt for photocatalytic H2 evolution.


 Please wait while we load your content...
Please wait while we load your content...