An ESIPT-based fluorescent switch with AIEE, solvatochromism, mechanochromism and photochromism†
Abstract
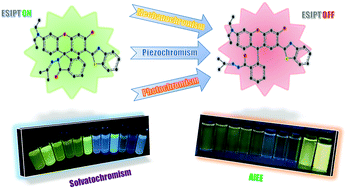
In this study, we report a newly synthesized molecule 1via linking a benzothiazole to rhodamine, which could exhibit AIEE, unique acidichromism and solvatochromism in solution, fascinating photochromism in a polymer matrix, intriguing mechanochromism in solid state and remarkable piezochromism in the crystal. 1 is a rarely reported organic molecule, which is sensitive to multiple stimuli such as acid/base, force, pressure, light and solvent polarity due to its distinct intramolecular hydrogen bonding and spiro-lactam structure. 1 demonstrated AIEE property in a THF/H2O system because of the water-induced aggregation and aggregation-suppressed nonradiative relaxation of the excited states. In the solution, 1 afforded yellowish-green emission at 520 nm (keto emission) in a non-polar solvent and blue emission at 447 nm (enol emission) in a polar solvent. Surprisingly, a blue shift in the emission peak was observed in a DCM solution upon treatment with acid or base, which is quite different from that of traditional rhodamine. In the solid state, the emission color of 1 changed from yellowish-green to red upon grinding or applying hydrostatic pressure, owing to the force-induced ring-opening reaction. In the polymer matrix, 1 manifested reversible photochromic behaviour and could serve as a good photoprinting material. This study is expected to contribute to the development of multistimuli-responsive fluorescent switch with multiple functions in different states.
- This article is part of the themed collection: Recent Progress on Aggregation-Induced Emission


 Please wait while we load your content...
Please wait while we load your content...