Different crystallographic sodium manganese oxides for capacitive deionization: performance comparison and the associated mechanism†
Abstract
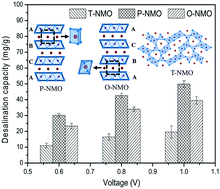
Sodium manganese oxide (NMO) has been proved experimentally to be an effective material for capacitive deionization (CDI). NMO exists in several crystallographic forms, which differ in the microstructure with tunnels or layered channels in their framework for Na+ diffusion and storage. In order to elucidate the dependence of the CDI performance of NMO on its crystal structure, both the tunnel NMO (T-NMO) and the layered NMO including the P2 group (P-NMO) and O3 group (O-NMO) were prepared and tested as electrode materials. The desalination test showed that although all NMOs displayed excellent cycling stability, the desalination capacity varied enormously among the different crystallographic NMOs, which kept the order P-NMO > O-NMO > T-NMO as the voltage ranged from 0.6 to 1.0 V. The desalination processes of the different crystallographic NMOs were revealed to be dominated by faradaic intercalation reactions and their respective desalination capacities were ascribed to the varied amounts of the Mn3+/Mn4+ couple involved in the faradaic reactions which were associated with the disparate Na+ available equivalents, diffusion channels and migration paths. Consequently, this study suggested that P-NMO has the most considerable application in the CDI system.


 Please wait while we load your content...
Please wait while we load your content...