Enhanced Cr(vi) immobilization on goethite derived from an extremely acidic environment†
Abstract
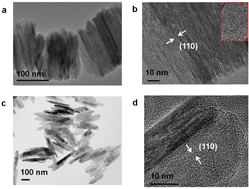
Iron (hydr)oxides play an indispensable role in the immobilization of heavy metal ions in environmental geochemical processes. As is well-known, the mineralization process of iron (hydr)oxides strongly depends on their surrounding environment. Goethite, a common iron oxide hydrate, is often naturally mineralized or artificially prepared in an alkaline environment; therefore, most of the studies are limited to alkaline environment-derived goethite; moreover, although the mineralization of goethite often takes place in an extremely acidic environment (e.g., acid mine drainage), little information is available on the heavy metal ion immobilization ability of acidic environment-derived goethite. In this study, we investigated the mineralization of goethite in an extremely acidic environment (GA) and compared its Cr(VI) immobilization performance with that of the alkaline-derived counterpart (GB). It was interestingly found that the maximum amount and apparent rate of Cr(VI) immobilization for GA were 0.124 mg m−2 and 15.68 m−2 mg−1 min−1, 2.4 times and 2.9 times those of GB, respectively. This outstanding Cr(VI) immobilization performance of GA was attributed to its higher surface hydroxyl concentration, as evidenced by the in situ IR spectra and X-ray photoelectron spectra. The higher surface hydroxyl concentration of GA originated from the preferential growth of its {021} facet with higher –OH group density in an acidic environment, resulting in its higher facet proportion of {021}/{110}. Moreover, we carefully identified the coordination mode of Cr(VI) immobilized on the GA and GB surface using Cr K-edge near-edge structure spectroscopy and discovered the formation of both monodentate mononuclear and bidentate binuclear complexes. These findings shed light on the influence of acidity on the mineralization and heavy metal pollutant adsorption performance of goethite and also provide a method for the preparation of goethite with high heavy metal ion immobilization performance.


 Please wait while we load your content...
Please wait while we load your content...