Atomic interface effect of a single atom copper catalyst for enhanced oxygen reduction reactions†
Abstract
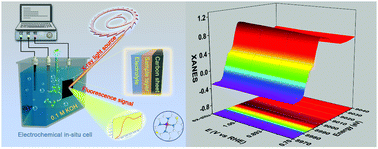
The regulation of catalytic activity in the oxygen reduction reaction (ORR) is significant to the development of metal–air batteries and other oxygen involving energy conversion devices. Herein, we propose an atomic interface strategy to construct a single atom copper catalyst (denoted as Cu-SA/SNC) which exhibits enhanced ORR activity with a half-wave potential of 0.893 V vs. RHE in alkaline media. Moreover, synchrotron-radiation-based X-ray absorption fine structure (XAFS) investigations together with density functional theory (DFT) calculations reveal that the isolated bond-shrinking low-valence Cu (+1)–N4–C8S2 atomic interface moiety serves as an active site during the ORR process, and the synergistic mechanism between the Cu species and the carbon matrix at the atomic interface plays a critical role in boosting the ORR efficiency, by adjusting the reaction free energy of intermediate adsorption. This atomic interface concept may provide an alternative methodology for the rational design of advanced oxygen electrode materials and new probability to improve their catalytic performance.


 Please wait while we load your content...
Please wait while we load your content...