An amorphous nickel boride-modified ZnxCd1−xS solid solution for enhanced photocatalytic hydrogen evolution
Abstract
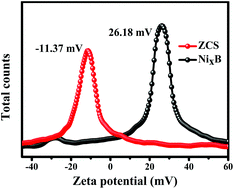
In this work, the rational design of amorphous NixB as a co-catalyst for the modification of ZnxCd1−xS was achieved. First, NixB was prepared by the redox method and ZnxCd1−xS was synthesized by the hydrothermal method. Then, the electrostatic self-assembly method was used to complete the coupling of NixB and ZnxCd1−xS. The experimental results of H2 production showed that when the mass ratio (wt%) of NixB in NixB/ZnxCd1−xS was 7 wt%, it had the highest activity, and the hydrogen production could reach 1521 μmol under irradiation with light for 5 h. The hydrogen production activity of the composite catalyst was 4.75 times higher than that of pure ZnxCd1−xS. The improvement in the photocatalytic activity can be attributed to the following aspects: (i) UV-vis DRS showed that the addition of NixB increased the absorption of visible light; (ii) the PL, TRPL, IT, EIS and LSV experiments further proved that the composite catalyst had excellent photoelectrochemical properties; (iii) the zeta potential results indicated that the electrostatic attraction between NixB and ZnxCd1−xS provided favorable conditions for the rapid transfer of electrons. This work shows that amorphous NixB has a prominent effect on the modification of ZnxCd1−xS, which provides more ways for the study of photocatalytic H2 evolution.


 Please wait while we load your content...
Please wait while we load your content...