Synthesis, characterisation and redox properties of anti-bimetallic permethylpentalene complexes†
Abstract
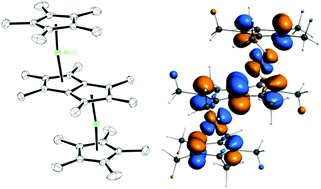
We report a series of anti-bimetallic transition metal complexes based on the permethylpentanyl (Pn*, C8Me6) ligand: anti-(MCpR5)2Pn* (where M = Fe, Co and Ni and R = H or Me). Compounds (FeCp*)2Pn* (1), (CoCp*)2Pn* (2), (NiCp*)2Pn* (3), (CoCp)2Pn* (4) and (NiCp)2Pn* (5) were fully characterised by NMR spectroscopy, X-ray crystallography, DFT calculations and cyclic voltammetry. All anti-(MCpR5)Pn* structures have diamagnetic ground states. 1, 2 and 4 have isolated non-degenerate ground states with closed shell configurations. A study of the magnetic properties of the dinickel complexes 3 and 5 reveal Boltzmann population of a Curie triplet state in the solution. Cyclic voltammetry reveals multiple reversible or quasi-reversible redox couples for all the dinuclear complexes.
- This article is part of the themed collection: Challenges in organometallic & coordination chemistry: in celebration of Geoff Cloke’s 65th birthday


 Please wait while we load your content...
Please wait while we load your content...