Synthesis of magnesium complexes of ionic liquids with highly coordinating anions†
Abstract
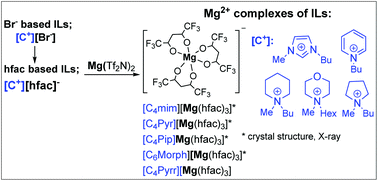
Magnesium(II) complexes, [Mg2+(hfac−)3][Cation+], were prepared as solids from hydrophobic hexafluoroacetylacetonate ionic liquids ([Cation+][hfac−] ILs) and Mg(Tf2N)2. 1-Butyl-3-methylimidazolium ([C4mim]), N-butylpyridinium ([C4Pyr]), N-butyl-N-methylpiperidinium ([C4Pip]), N-hexyl-N-methylmorpholinium ([C6Morp]) and N-butyl-N-methylpyrrolidinium ([C4pyrr]) were used as cationic cores. The [C4Pip][hfac], [C4Pyr][hfac] and [C6Morp][hfac] ILs were prepared for the first time. New Mg(II) complexes, [C4mim][Mg(hfac)3], [C4Pip][Mg(hfac)3], [C4Pyr][Mg(hfac)3], [C6Morp][Mg(hfac)3] and [C4Pyrr][Mg(hfac)3], were obtained from the [hfac] based ILs. The crystal structures of the novel Mg(II) complexes show the coordination of three [hfac] anions to the Mg2+ ion through the two oxygen atoms of each [hfac] anion.


 Please wait while we load your content...
Please wait while we load your content...