Coexistence of three types of sodium motion in double molybdate Na9Sc(MoO4)6: 23Na and 45Sc NMR data and ab initio calculations
Abstract
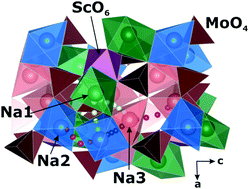
The rechargeable Na-ion batteries attract much attention as an alternative to the widely used but expensive Li-ion batteries. The search for materials with high sodium diffusion is important for the development of solid state electrolytes. We present the results of experimental and ab initio studies of the Na-ion diffusion mechanism in Na9Sc(MoO4)6. The ion conductivity reaches the value of 3.6 × 10−2 S cm−1 at T ∼ 850 K. The 23Na and 45Sc NMR data reveal the coexistence of three different types of Na-ion motion in the temperature range from 300 to 750 K. They are activated at different temperatures and are characterized by substantially different dynamics parameters. These features are confirmed by ab initio calculations of activation barriers for sodium diffusion along various paths.


 Please wait while we load your content...
Please wait while we load your content...