Fabrication of zwitterionic and pH-responsive polyacetal dendrimers for anticancer drug delivery†
Abstract
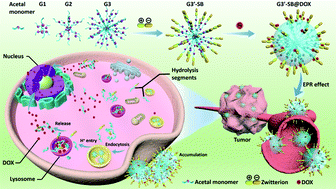
Dendrimer micelles are considered to be the most promising synthetic macromolecules for biomedical applications, but their instability in the blood circulation and undesired release at a non-targeted location have become the major concerns for recent research. Herein, zwitterionic sulfobetaine (SB) functionalized polyacetal dendrimers were fabricated through the alternant aza-Michael addition and thiol–ene click reactions. On account of the acid-labile characteristics of acetal segments and charge reversal effects of sulfobetaine moieties, these uniform dendrimers could form biocompatible and biodegradable micelles in aqueous solutions, which present excellent structural stability, good resistivity to protein absorption and high internalization efficiency for a long period. More importantly, effected by the charge shielding of zwitterions on the surface of polyacetal dendrimers, these DOX-loaded nanoparticles exhibited extremely significant pH-responsive drug release behaviors and remarkable anticancer activity. Thus, we believe that this work not only sheds new light on the application of polyacetal dendrimers but also paves a better way for the development of robust on-demand anti-cancer nanosystems with significant potential for clinical translation.


 Please wait while we load your content...
Please wait while we load your content...