Thioether- and sulfone-functionalized dibenzopentalenes as n-channel semiconductors for organic field-effect transistors†
Abstract
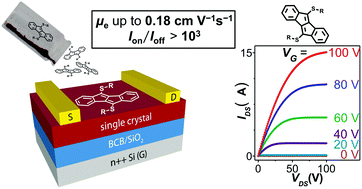
Dibenzo[a,e]pentalenes (DBPs) are promising candidates to be used as ambipolar or n-type semiconductors in organic field-effect transistors (OFETs). For n-channel conduction, low LUMO energy levels are required. Furthermore, a close molecular packing in the solid state is advantageous. Here we present thioether-functionalized DBPs with LUMO energies down to −3.47 eV as well as a DBP sulfone with a LUMO energy of −3.94 eV. X-ray crystallography revealed 1-D π-stacking or herringbone packing in the solid state with close intramolecular distances between the DBP cores. Two thio-DBPs and the DBP sulfone showed n-channel conduction in FET measurements on well-aligned crystals with electron mobilities of up to 0.18 cm2 V−1 s−1, to date the highest reported values for n-channel conduction in DBP derivatives. The short synthetic route will in the future allow for facile access to a variety of thio- and sulfone-DBPs for n-channel or ambipolar semiconduction.


 Please wait while we load your content...
Please wait while we load your content...