Single ion conducting lithium sulfur polymer batteries with improved safety and stability†
Abstract
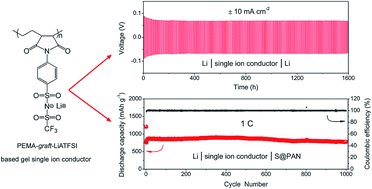
Li–S secondary batteries use lithium metal as the anode. The safety hazard arising from the Li dendrite formation on the metal surface presents a formidable challenge that has hindered the technology from practical applications for many years. It has been confirmed that tiny and random lithium deposition takes place at the ion depletion layer on the surface of lithium metal. The time required to reach the ion depletion layer can be quantified using Sand's equation, in which the time is inversely proportional to the transference number of anions (t−). Therefore, restricting the mobility of anions enables avoidance of ion depletion. In this study, lithium 4-aminophenylsulfonyl(trifluoromethylsulfonyl)imide (LiATFSI) is grafted with poly(ethylene-alt-maleic anhydride) (PEMA, Mw = 100 000–500 000) using a cyclic imide to form a single ion conducting polymer electrolyte (PEMA-graft-LiATFSI). The polymer electrolyte membrane made of PEMA-graft-LiATFSI is capable of withstanding a high current density of ±50 mA cm−2 (normalized to the surface area of the lithium disk) in a lithium symmetric cell. More remarkably, the metallic luster of the lithium foil remains essentially intact even after a galvanostatic cycling test with a current density of ±10 mA cm−2 for over 1600 hours, suggesting that the membrane can effectively suppress Li dendrite formation and thus pave a way to use lithium metal directly as the anode material with sufficient energy capacity and good safety. The lithium–sulfur battery assembled with the membrane as the electrolyte as well as the separator delivered a stable capacity of 780.8 mA h g−1 after 1000 cycles at 1C. This work demonstrates the necessity and fundamental importance of single ion conducting electrolyte membranes for achieving safe and stable performance with high energy density lithium metal secondary batteries.


 Please wait while we load your content...
Please wait while we load your content...