Interaction of oxygen with halide perovskites†
Abstract
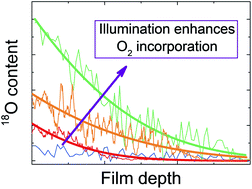
We systematically investigate the consequences of exposing halide perovskites to an oxygen-containing atmosphere, in particular methylammonium lead iodide, the archetypal compound used as photo-absorber in perovskite solar cells. For this purpose, we study oxygen solubility and global reaction with oxygen both in the dark and under light, and we refer to the kinetics in terms of surface reaction and bulk diffusion. As thermodynamics reveals, the material is unstable against oxygen, primarily because of the large driving force of water formation. While under light the material quickly degrades, in the dark the surface reaction kinetics – not the bulk transport – is very sluggish and keeps it metastable. For the same reason, oxygen incorporation into the lattice is negligible in the dark. On illumination, an accelerated oxygen in-diffusion occurs (as shown by 18O incorporation experiments) that severely modifies the electronic and ionic conductivities in the way that is expected for an acceptor dopant. Lastly, we investigate the impact of cation as well as anion mixing on the degradation and stress the necessity of using encapsulation during solar cell operation.


 Please wait while we load your content...
Please wait while we load your content...