Nanoporous AuPt and AuPtAg alloy co-catalysts formed by dewetting–dealloying on an ordered TiO2 nanotube surface lead to significantly enhanced photocatalytic H2 generation†
Abstract
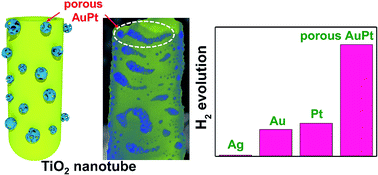
Effective co-catalysts are of key importance for photocatalytic H2 generation from aqueous environments. AuPt (metastable) alloys are an attractive co-catalyst candidate due to the synergistic electronic and chemical interaction of the constituents in the charge transfer and H2 evolution processes. Here we introduce the fabrication of AuPt alloy nanoparticles with nanoporosity (pore size of 2–5 nm) on spaced TiO2 nanotubes. By dewetting a layered AgAuPt coating, we form AuPtAg alloy nanoparticles. From these alloys, Ag can selectively be dissolved leading to the desired nanoporous AuPt alloy particles with diameters in the range of 10–70 nm deposited as a gradient on the TiO2 nanotubes. A significant enhancement of photocatalytic H2 generation is obtained compared to that with the same loading of monometallic or nonporous alloys. The nanoporous AuPt particles not only provide a large surface area to volume ratio (and are thus more effective) but also show the intrinsic synergy of a AuPt alloy for H2 generation.


 Please wait while we load your content...
Please wait while we load your content...