A new type of cyclic silicone additive for improving the energy density and power density of Li–O2 batteries†
Abstract
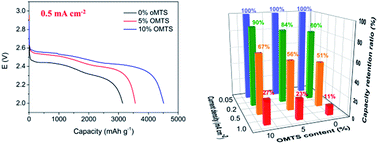
In this work, a novel electrolyte additive, octamethylcyclotetrasiloxane (OMTS), is applied to Li–O2 batteries to increase their practical discharge capacity and also their rate capability. By adding OMTS into a tetraethylene glycol dimethoxyethane (TEGDME)-based electrolyte, the solubility of oxygen increases significantly, and the ionic conductivity and viscosity remain the same. 7Li nuclear magnetic resonance (NMR) spectra show that the 7Li peak shifts downfield when adding OMTS to the electrolyte, indicating the increment of the solvation of Li+ in the electrolyte. The electrochemical tests show that, with an optimal OMTS content (10 vol% OMTS), the cell displays a high discharge capacity of 6778 mA h g−1 at 0.05 mA cm−2. Its capacity retention is more than double that of the cell with no OMTS additive at a large current density of 1 mA cm−2. Further NMR and Li2O2 yield measurements during discharge indicate that the OMTS additive does not alter the discharge product, or compromise the stability of the TEGDME electrolyte. The great increment in energy density and power density could be attributed to the high oxygen solubility and increment of solvated Li+, leading to the formation of more nodular products, as observed by scanning electron microscopy (SEM).


 Please wait while we load your content...
Please wait while we load your content...