Assembly modes of hexaphenylalanine variants as function of the charge states of their terminal ends†
Abstract
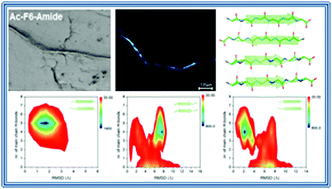
The ability of peptides to self-assemble represents a valuable tool for the development of biomaterials of biotechnological and/or biomedical interest. Diphenylalanine homodimer (FF) and its analogues are among the most promising systems in this field. The longest Phe-based building block hitherto characterized is pentaphenylalanine (F5). We studied the aggregation propensity and the structural/morphological features of assemblies of zwitterionic hexaphenylalanine H+-F6-O− and of three variants characterized by different charged states of the terminal ends (Ac-F6-Amide, H+-F6-Amide and Ac-F6-O−). As previously observed for PEGylated hexaphenylalanine (PEG8-F6), all F6 variants show a strong tendency to form β-rich assemblies in which the structural motif is constituted by antiparallel β-strands in the cross-β framework. Extensive replica exchange molecular dynamics simulations carried out on a pairs of F6 peptides indicate that the antiparallel β-structure of the final assemblies is likely dictated by the preferred association modes of the individual chains in the very early stages of the aggregation process. Our data suggest that even very small F6 peptides are properly pre-organized and prone to the build-up of the final assembly.


 Please wait while we load your content...
Please wait while we load your content...