Effects of additives on the viscoelastic responses of cationic gemini surfactant solutions†
Abstract
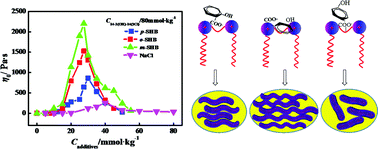
Several additives, including inorganic (NaCl) and organic salts (derivatives of benzoate), were added into aqueous solutions of a gemini cationic surfactant, 2-hydroxypropyl-1,3-bis (myristyldimethylammonium chloride) (abbreviated as 14-3(OH)-14(2Cl)). The mixed systems were investigated using rheological measurement, cryo-TEM and 1H NMR analysis. The results showed that addition of salts induced rich aggregate morphologies in the 14-3(OH)-14(2Cl)/salt systems. The influence of an inorganic salt on the viscoelasticity of 14-3(OH)-14(2Cl) solutions is much weaker than that of organic salts. Furthermore, the ability of three organic salts in enhancing the viscoelasticity of 14-3(OH)-14(2Cl) solutions is in the order sodium m-hydroxybenzoate > sodium o-hydroxybenzoate > sodium p-hydroxybenzoate. The different roles of these organic salt isomers arise from the different types of hydrogen bonding formed between 14-3(OH)-14(2Cl) and the organic counter ions.


 Please wait while we load your content...
Please wait while we load your content...