Temperature-regulated protein adsorption on a PNIPAm layer†
Abstract
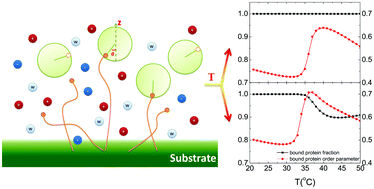
In immunosensors, antibody orientation is a key factor that determines the sensitivity of a device. To date much effort has been devoted to exploring strategies for the direct control of the orientation of antibodies immobilized on a bioactive surface, but less attention has been paid to controlling the orientation of intermediate proteins (though usually used when immobilizing antibodies), which may greatly limit the sensitivity of immunological activities. Therefore, it is of great significance to seek novel methods for controlling protein orientation. Here, we design a new strategy for controlling protein orientation. The main idea is to bind proteins to a ligand-functionalized poly(N-isopropylacrylamide) (PNIPAm) layer, and then the protein orientation can be mediated by environmental temperature. The theory predicts that the protein orientation can show unexpected triple-thermo-responsive behavior. Based on the fraction of ligand adsorbed by the protein, the reponsive behavior can be either complete adsorption or partial adsorption, which is determind by the polymer's surface coverage and the protein's properties. We expect that the present strategy can enrich the methods for controlling intermediate protein orientation and can guide the design of novel immunosensors with superior sensitivity.


 Please wait while we load your content...
Please wait while we load your content...