Separately enhanced dual emissions of the amphiphilic derivative of 2-(2′-hydroxylphenyl) benzothiazole by supramolecular complexation†
Abstract
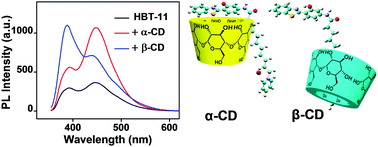
Here, we report separately enhanced dual emissions of the amphiphilic derivative of 2-(2′-hydroxyphenyl)benzothiazole (denoted as HBT-11) by supramolecular complexation with cyclodextrins (CDs). When dispersed in water, HBT-11 shows two relatively weak emission bands, which can be assigned to the emissions of enol- and keto-forms, the two tautomers, owing to excited-state intramolecular proton transfers. Upon the addition of α-CD and β-CD, the keto- and enol-emissions, respectively, are separately enhanced; the enhancement effect is due to the formation of HBT-11/α-CD and HBT-11/β-CD complexes through multiple hydrogen bonding and host–guest interactions, respectively. It is worth to note that the keto-emission caused by the complex of HBT-11/α-CD has a much shorter wavelength compared with that of the aggregates formed by pure HBT-11. To the best of our knowledge, this is the first time that a study on keto-emission of the isolated HBT chromophore has been reported.


 Please wait while we load your content...
Please wait while we load your content...