A quantitative reactivity scale for electrophilic fluorinating reagents†
Abstract
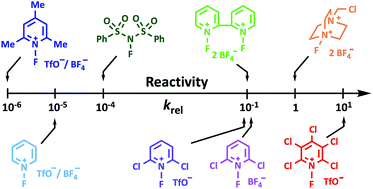
Electrophilic N–F fluorination agents underpin the introduction of fluorine in aliphatic systems across drug and academic research. The choice of N–F reagent is currently determined through empirical experimentation in the absence of quantitative values for electrophilicities. Here we report an experimentally-determined kinetic reactivity scale for ten N–F fluorinating reagents, including Selectfluor™, NFSI, Synfluor™ and several N-fluoropyridinium salts, in CH3CN. The reactivity scale, which covers eight orders of magnitude, employs para-substituted 1,3-diaryl-1,3-dicarbonyl derivatives to measure relative and absolute rate constants. The para-substituted 1,3-diaryl-1,3-dicarbonyl scaffold delivers a convenient, sensitive spectrophotometric reporter of reactivity that also led to the discovery of a unique form of tautomeric polymorphism.
- This article is part of the themed collection: Editor's choice - Paolo Melchiorre


 Please wait while we load your content...
Please wait while we load your content...