Replacing H+ by Na+ or K+ in phosphopeptide anions and cations prevents electron capture dissociation†
Abstract
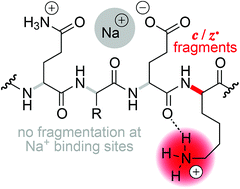
By successively replacing H+ by Na+ or K+ in phosphopeptide anions and cations, we show that the efficiency of fragmentation into c and z˙ or c˙ and z fragments from N–Cα backbone bond cleavage by negative ion electron capture dissociation (niECD) and electron capture dissociation (ECD) substantially decreases with increasing number of alkali ions attached. In proton-deficient phosphopeptide ions with a net charge of 2−, we observed an exponential decrease in electron capture efficiency with increasing number of Na+ or K+ ions attached, suggesting that electrons are preferentially captured at protonated sites. In proton-abundant phosphopeptide ions with a net charge of 3+, the electron capture efficiency was not affected by replacing up to four H+ ions with Na+ or K+ ions, but the yield of c, z˙ and c˙, z fragments from N–Cα backbone bond cleavage generally decreased next to Na+ or K+ binding sites. We interpret the site-specific decrease in fragmentation efficiency as Na+ or K+ binding to backbone amide oxygen in competition with interactions of protonated sites that would otherwise lead to backbone cleavage into c, z˙ or c˙, z fragments. Our findings seriously challenge the hypothesis that the positive charge responsible for ECD into c, z˙ or c˙, z fragments can generally be a sodium or other metal ion instead of a proton.
- This article is part of the themed collection: 2018 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...