Bifunctional iminophosphorane catalysed enantioselective sulfa-Michael addition of alkyl thiols to alkenyl benzimidazoles†
Abstract
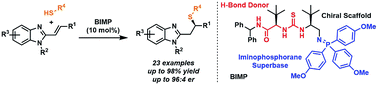
The first enantioselective sulfa-Michael addition of alkyl thiols to alkenyl benzimidazoles, enabled by a bifunctional iminophosphorane (BIMP) organocatalyst, is described. The iminophosphorane moiety of the catalyst provides the required basicity to deprotonate the thiol nucleophile while the chiral scaffold and H-bond donor control facial selectivity. The reaction is broad in scope with respect to the thiol and benzimidazole reaction partners with the reaction proceeding in up to 98% yield and 96 : 4 er.


 Please wait while we load your content...
Please wait while we load your content...