CuCl/TMEDA/nor-AZADO-catalyzed aerobic oxidative acylation of amides with alcohols to produce imides†
Abstract
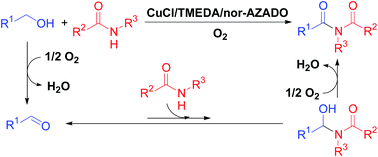
Although aerobic oxidative acylation of amides with alcohols would be a good complement to classical synthetic methods for imides (e.g., acylation of amides with activated forms of carboxylic acids), to date, there have been no reports on oxidative acylation to produce imides. In this study, we successfully developed, for the first time, an efficient method for the synthesis of imides through aerobic oxidative acylation of amides with alcohols by employing a CuCl/TMEDA/nor-AZADO catalyst system (TMEDA = teramethylethylendiamine; nor-AZADO = 9-azanoradamantane N-oxyl). The proposed acylation proceeds through the following sequential reactions: aerobic oxidation of alcohols to aldehydes, nucleophilic addition of amides to the aldehydes to form hemiamidal intermediates, and aerobic oxidation of the hemiamidal intermediates to give the corresponding imides. This catalytic system utilizes O2 as the terminal oxidant and produces water as the sole by-product. An important point for realizing this efficient acylation system is the utilization of a TMEDA ligand, which, to the best of our knowledge, has not been employed in previously reported Cu/ligand/N-oxyl systems. Based on experimental evidence, we consider that plausible roles of TMEDA involve the promotion of both hemiamidal oxidation and regeneration of an active CuII–OH species from a CuI species. Here promotion of hemiamidal oxidation is particularly important. Employing the proposed system, various types of structurally diverse imides could be synthesized from various combinations of alcohols and amides, and gram-scale acylation was also successful. In addition, the proposed system was further applicable to the synthesis of α-ketocarbonyl compounds (i.e., α-ketoimides, α-ketoamides, and α-ketoesters) from 1,2-diols and nucleophiles (i.e., amides, amines, and alcohols).


 Please wait while we load your content...
Please wait while we load your content...