Isolable iodosylarene and iodoxyarene adducts of Co and their O-atom transfer and C–H activation reactivity†
Abstract
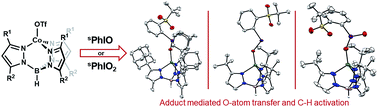
We report an unusual series of discrete iodosyl- and iodoxyarene adducts of Co. The formation of these adducts was confirmed by a suite of techniques including single crystal X-ray diffraction. The reactivity of these adducts with O-atom acceptors and an H-atom donor has been investigated with particular focus on elucidating mechanistic details. Detailed kinetic analysis allows for discrimination between proposed oxo and adduct mediated mechanisms. In particular, these reactions have been interrogated by competition experiments with isotopically labelled mixtures which shows that all of the studied adducts display a large KIE. These studies suggest different mechanisms may be relevant depending on subtle substituent changes in the adduct complexes. Reactivity data are consistent with the involvement of a transient oxo complex in one case, while the two other systems appear to react with substrates directly as iodosyl- or iodoxyarene adducts. These results support that reactivity typically ascribed to metal-oxo complexes, such as O-atom transfer and C–H activation, can also be mediated by discrete transition metal iodosyl- or iodoxyarene adducts that are frequent intermediates in the generation of oxo complexes. The influence of additional Lewis acids such as Sc3+ on the reactivity of these systems has also been investigated.


 Please wait while we load your content...
Please wait while we load your content...