Aryl transition metal chemical warheads for protein bioconjugation
Abstract
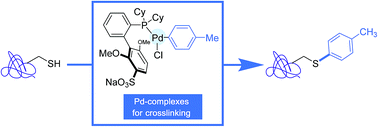
The past seven years have witnessed the burgeoning of protein bioconjugation reactions highlighting aryl transition metal reagents as coupling partners. This new bioorthogonal organometallic chemistry, which sets the scene for stoichiometric processes in place of the catalytic procedures that developed in parallel, already enabled the forging of C–S and C–C bonds onto protein substrates, respectively in their native state or equipped with pre-installed non-natural terminal alkene or alkyne appendages. Although not yet applied to proteins, related transformations pointing to the creation of C–N bonds have, in addition, just been disclosed by targeting peptide lysine residues. Central to this research was the selection of ligands attached to the transition metal, in order to confer to metal complexes, not only their stability in aqueous medium, but also the desired chemoselectivity. We summarize here this body of work, which has already put in the limelight elaborated palladium and gold complexes equipped with biologically relevant appendages, such as fluorescent and affinity tags, as well as drug molecules. This research holds much promise, not only for the study of proteins themselves, but also for the design of new protein-based biotherapeutics, such as protein-drug conjugates or constrained analogs resulting from macrocyclisation reactions.


 Please wait while we load your content...
Please wait while we load your content...