Predicting the DNP-SENS efficiency in reactive heterogeneous catalysts from hydrophilicity†
Abstract
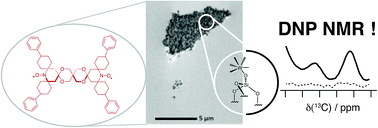
Identification of surfaces at the molecular level has benefited from progress in dynamic nuclear polarization surface enhanced NMR spectroscopy (DNP SENS). However, the technique is limited when using highly sensitive heterogeneous catalysts due to secondary reaction of surface organometallic fragments (SOMFs) with stable radical polarization agents. Here, we observe that in non-porous silica nanoparticles (NPs) (dparticle = 15 nm) some DNP enhanced NMR or SENS characterizations are possible, depending on the metal-loading of the SOMF and the type of SOMF substituents (methyl, isobutyl, neopentyl). This unexpected observation suggests that aggregation of the nanoparticles occurs in non-polar solvents (such as ortho-dichlorobenzene) leading to (partial) protection of the SOMF inside the interparticle space, thereby preventing reaction with bulky polarization agents. We discover that the DNP SENS efficiency is correlated with the hydrophilicity of the SOMF/support, which depends on the carbon and SOMF concentration. Nitrogen sorption measurements to determine the BET constant (CBET) were performed. This constant allows us to predict the aggregation of silica nanoparticles and consequently the efficiency of DNP SENS. Under optimal conditions, CBET > 60, we found signal enhancement factors of up to 30.


 Please wait while we load your content...
Please wait while we load your content...