Using stereoretention for the synthesis of E-macrocycles with ruthenium-based olefin metathesis catalysts†
Abstract
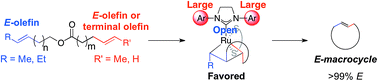
The synthesis of E-macrocycles is achieved using stereoretentive, Ru-based olefin metathesis catalysts supported by dithiolate ligands. Kinetic studies elucidate marked differences in activity among the catalysts tested, with catalyst 4 providing meaningful yields of products in much shorter reaction times than stereoretentive catalysts 2 and 3. Macrocycles were generated with excellent selectivity (>99% E) and in moderate to high yields (47–80% yield) from diene starting materials bearing two E-configured olefins. A variety of rings were constructed, ranging from 12- to 18-membered macrocycles, including the antibiotic recifeiolide.
- This article is part of the themed collection: Editor’s choice – Andrei Yudin


 Please wait while we load your content...
Please wait while we load your content...