Controlling destructive quantum interference in tunneling junctions comprising self-assembled monolayers via bond topology and functional groups†
Abstract
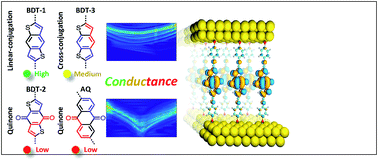
Quantum interference effects (QI) are of interest in nano-scale devices based on molecular tunneling junctions because they can affect conductance exponentially through minor structural changes. However, their utilization requires the prediction and deterministic control over the position and magnitude of QI features, which remains a significant challenge. In this context, we designed and synthesized three benzodithiophenes based molecular wires; one linearly-conjugated, one cross-conjugated and one cross-conjugated quinone. Using eutectic Ga–In (EGaIn) and CP-AFM, we compared them to a well-known anthraquinone in molecular junctions comprising self-assembled monolayers (SAMs). By combining density functional theory and transition voltage spectroscopy, we show that the presence of an interference feature and its position can be controlled independently by manipulating bond topology and electronegativity. This is the first study to separate these two parameters experimentally, demonstrating that the conductance of a tunneling junction depends on the position and depth of a QI feature, both of which can be controlled synthetically.
- This article is part of the themed collection: Celebrating the Chemical Sciences in India - Leaders in the Field Symposium 2020


 Please wait while we load your content...
Please wait while we load your content...