Organocatalytic reductive coupling of aldehydes with 1,1-diarylethylenes using an in situ generated pyridine-boryl radical†
Abstract
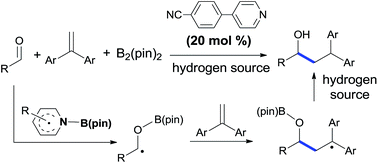
A pyridine-boryl radical promoted reductive coupling reaction of aldehydes with 1,1-diarylethylenes has been established via a combination of computational and experimental studies. Density functional theory calculations and control experiments suggest that the ketyl radical from the addition of the pyridine-boryl radical to aldehydes is the key intermediate for this C–C bond formation reaction. This metal-free reductive coupling reaction features a broad substrate scope and good functional compatibility.


 Please wait while we load your content...
Please wait while we load your content...