Diastereo- and enantioselective additions of α-nitro esters to imines for anti-α,β-diamino acid synthesis with α-alkyl-substitution†
Abstract
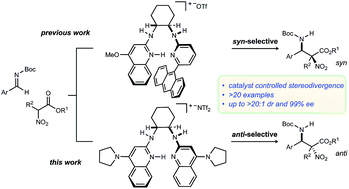
The discovery that a C2-symmetric bis(AMidine) [BAM] catalyst promotes an anti-selective addition of α-substituted α-nitro esters to imines is described, providing α-substituted α,β-diamino ester products with high diastereo- and enantioselectivity. When compared to the function of a BAM catalyst reported previously, the pair offer a rare example of diastereodivergence using a bifunctional Brønsted acid–base organocatalyst.


 Please wait while we load your content...
Please wait while we load your content...