A water-soluble supramolecular complex that mimics the heme/copper hetero-binuclear site of cytochrome c oxidase†‡
Abstract
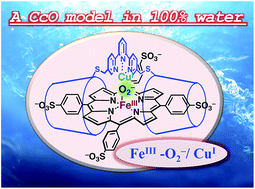
In mitochondria, cytochrome c oxidase (CcO) catalyses the reduction of oxygen (O2) to water by using a heme/copper hetero-binuclear active site. Here we report a highly efficient supramolecular approach for the construction of a water-soluble biomimetic model for the active site of CcO. A tridentate copper(II) complex was fixed onto 5,10,15,20-tetrakis(4-sulfonatophenyl)porphinatoiron(III) (FeIIITPPS) through supramolecular complexation between FeIIITPPS and a per-O-methylated β-cyclodextrin dimer linked by a (2,2′:6′,2′′-terpyridyl)copper(II) complex (CuIITerpyCD2). The reduced FeIITPPS/CuITerpyCD2 complex reacted with O2 in an aqueous solution at pH 7 and 25 °C to form a superoxo-type FeIII–O2−/CuI complex in a manner similar to CcO. The pH-dependent autoxidation of the O2 complex suggests that water molecules gathered at the distal Cu site are possibly involved in the FeIII–O2−/CuI superoxo complex in an aqueous solution. Electrochemical analysis using a rotating disk electrode demonstrated the role of the FeTPPS/CuTerpyCD2 hetero-binuclear structure in the catalytic O2 reduction reaction.


 Please wait while we load your content...
Please wait while we load your content...