Correlation between electrochemical properties and stress corrosion cracking of super 13Cr under an HTHP CO2 environment
Abstract
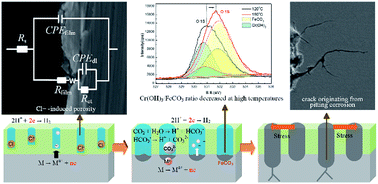
The susceptibility of super 13Cr steel to stress corrosion cracking (SCC) was assessed through slow strain rate testing in simulated formation water saturated with CO2 under a high-temperature and high-pressure (HTHP) environment. The evolution, morphology, and chemistry of fracture and corrosion products on the steel surface were evaluated using in situ electrochemical methods and surface analysis. Results indicate that the occurrence of pitting corrosion increases SCC susceptibility. At 150 °C, the degradation of a surface film induces pitting corrosion because of an increase in anodic processes. The presence of Cl− causes film porosity, and CO2 reduces the Cr(OH)3/FeCO3 ratio in the inner film, which further promotes Cl−-induced porosity.


 Please wait while we load your content...
Please wait while we load your content...