One-pot three component synthesis of 5-allyl-1,2,3-triazoles using copper(i) acetylides†
Abstract
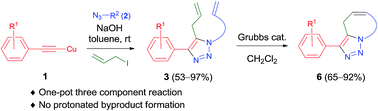
One-pot three-component reactions using copper(I) acetylide, azide, allyl iodide, and NaOH have been developed. The reactions proceed smoothly at room temperature to afford 5-allyl-1,2,3-triazoles, which can be further transformed into a variety of 1,2,3-triazole-fused bi-/tricyclic scaffolds. This method offers the most efficient, convenient, and practical route towards useful polycyclic scaffolds in moderate to excellent yields.


 Please wait while we load your content...
Please wait while we load your content...