Structural and impedance spectroscopy characteristics of BaCO3/BaSnO3/SnO2 nanocomposite: observation of a non-monotonic relaxation behavior
Abstract
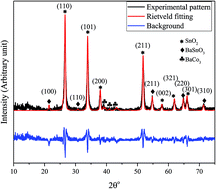
A BaCO3/BaSnO3/SnO2 nanocomposite has been prepared using a co-precipitation method without adding any additives. The prepared sample was characterized by using X-ray diffraction (XRD), high-resolution transmission electron microscopy (HRTEM), scanning electron microscopy (SEM), Fourier-transform infrared (FT-IR) spectroscopy, energy dispersive X-ray spectroscopy (EDS) and Raman spectroscopy. Detailed studies on the dielectric and electrical behavior (dielectric constant, complex impedance Z*, ac conductivity, and relaxation mechanisms) of the nanocomposite have been performed using the nondestructive complex impedance spectroscopy technique within the temperature range 150–400 K. The dielectric constant of the sample as a function of temperature showed the typical characteristics of a relaxor. The maximum dielectric constant value was observed to depend on frequency. The non-monotonic relaxation behavior of the prepared nanocomposite was evidenced from the spectra of loss tan, tan(δ). The relaxation kinetics was modeled using a non-Arrhenius model.


 Please wait while we load your content...
Please wait while we load your content...