Reductive cleavage of the N–O bond: elemental sulfur-mediated conversion of N-alkoxyamides to amides†
Abstract
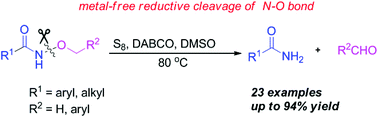
By treating with elemental sulfur in the presence of DABCO in DMSO, a variety of N-alkoxyamides were readily converted to amides in satisfactory to excellent yields via a metal-free reductive cleavage of the N–O bond. The mechanism of the reaction has been proposed based on the results of the control experiments, suggesting a process of cleavage of the N–O bond mediated by elemental sulfur, as well as the role played by DMSO in forming the amide in the product.


 Please wait while we load your content...
Please wait while we load your content...