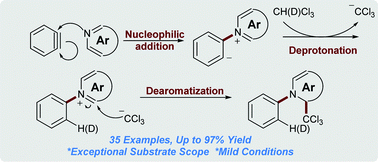
Abstract
The direct dearomatization reaction of isoquinolines and quinolines with chloroform has been accomplished through in situ electrophilic aryne activation and nucleophile formation. This transition metal-free protocol shows a broad scope of substrates, providing consistently high yields of medicinally-important dihydro(iso)quinoline derivatives. The utility of this method has been further demonstrated in the synthesis of deuterated analogues.


 Please wait while we load your content...
Please wait while we load your content...