An efficient protocol for the preparation of aldehydes/ketones and imines by an inorganic-ligand supported iron catalyst†
Abstract
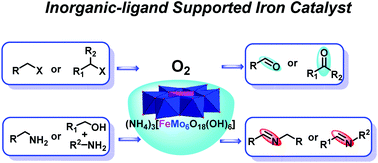
Oxidation is a powerful methodology that is widely used for the production of fine chemicals and pharmaceutical intermediates, however, this process often involves the use of toxic reagents and harsh reaction conditions. Herein we report an efficient and eco-friendly way to promote the aerobic oxidation of organic halides to aldehydes/ketones and oxidative coupling of amines to imines using an inorganic-ligand supported iron catalyst 1a (NH4)3[FeMo6O18(OH)6] with O2 (1 atm) as the sole oxidant. 1a is synthesized directly from cheap and commonly available (NH4)6Mo7O24·4H2O and Fe2(SO4)3, which consist of a pure inorganic framework built from a central FeIII core supported by six MoVIO6 inorganic scaffolds. The iron catalyst 1a exhibits better activity towards a wide range of substrates in the catalytic oxidation of halides and amines, and the use of toxic oxidants and potentially air/moisture sensitive and complicated organic ligands that are not commercially available is avoided. Owing to the robust inorganic framework, 1a shows good stability and reusability and the catalytic oxidation of halides and amines could be readily scaled up to gram-scale with little loss of catalytic activity, demonstrating the great potential of inorganic-ligand supported catalysts in catalytic chemical transformation.


 Please wait while we load your content...
Please wait while we load your content...