Reactivity of arynes toward functionalized alkenes: intermolecular Alder-ene vs. addition reactions†
Abstract
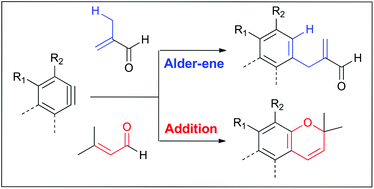
The selectivity between two different manifolds of reactions of arynes reacting with functionalized alkenes is described. Arynes generated from bis-1,3-diynes react with various trisubstituted and 1,1-disubstituted alkenes including methallyl amine, prenyl azide, and methacrylic acid, providing mainly addition products of the polar heteroatom functionalities over the Alder-ene products of the alkene segment. The selectivity, however, intricately depends on the substituent pattern of the alkene. Except for the most reactive 2-propenyl group-containing aldehyde, α,β-unsaturated aldehydes generally participated in an addition reaction, generating chromene derivatives.


 Please wait while we load your content...
Please wait while we load your content...
