Palladium-catalyzed carbonylation of thioacetates and aryl iodides for the synthesis of S-aryl thioesters†
Abstract
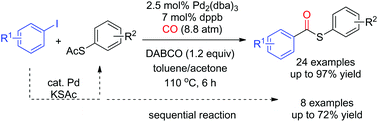
Thioesters were synthesized via palladium-catalyzed carbonylation of thioacetates and aryl iodides. S-Aryl thioacetates coupled with carbon monoxide and aryl iodides to afford the desired S-aryl thioesters in good yields. The reaction showed good functional group tolerance toward fluoro, chloro, ketone, ester, aldehyde, cyano, and nitro groups. The tandem reaction of the direct S-arylation of aryl iodides from potassium thioacetate (KSAc) and subsequent carbonylation of the intermediates S-aryl thioacetates provided S-aryl thioesters in moderate-to-good yields.


 Please wait while we load your content...
Please wait while we load your content...